
When simple activities such as walking or standing become a burden, you may feel that pain has taken control of your life. The Vertiflex® Procedure may help you take it back. Your leg and back pain might be the result of a condition called lumbar spinal stenosis (LSS), which can develop from normal wear and tear on your spine as you age. LSS is common in adults over the age of 60.
How does the Vertiflex Procedure work?
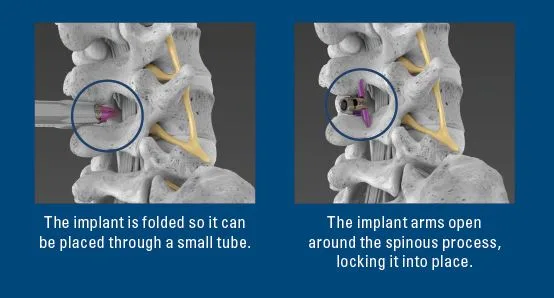
The Vertiflex® Procedure is a minimally invasive outpatient treatment. It uses a small implant that relieves pressure on the affected nerves. It was designed with patient safety and comfort in mind.The implant works by supporting your existing anatomy and is reversible, leaving all treatment options available in the future. The Vertiflex Procedure may not be right for everyone, as any treatment has associated risks. Ask your doctor if the Vertiflex Procedure is right for you.
Is Vertiflex Right for You?
Individuals who experience the following symptoms may be candidates
for the Vertiflex Procedure:
- Pain while walking
- Numbness or a “tingling” feeling in the legs, calves, or buttocks
- Weakness and/or loss of balance
- Aching, dull back pain spreading to the legs
- Decreased endurance during physical activities
- Pain relief is experienced when leaning/bending forward or sitting down
Contact Us for Leading Pain Management Treatments and Regenerative Orthopedic Solutions for Joint and Spine Pain
We look forward to seeing you. Please call us anytime between 8am and 4:30pm Monday through Friday, or complete this form to request an appointment.
If you use our form , one of our schedulers will contact you by phone within one business day to arrange a convenient time and date for your appointment. Online appointment requests received on Fridays may not be confirmed until the following business day (Monday, excluding holidays).

